Last Update 25/ 3/ 2010
in English/ in Esperanto/
in
Portuguese
Chemical oxidation and reduction reactions or redox reactions may occur
spontaneously when a material chemical species able to donate electron
contacts a chemical species able to receive electron. The electron donor
looses electron and is the reducing agent. The electron receiver is the
oxidizing agent. When the reducing agent is depleted the reaction stops.
If the oxidizing agent is depleted the reaction stops as well. However
the reaction will be restored after a new supply of the depleted species.
The reducing agent may loose more than one electron if there is oxidizing
agent to receive all the electrons and the oxidizing agent can receive
more than one electron if there is reducing agent to donate the electrons.
The study of the above theme is supported by universally accepted methods
and conventions.
When the above mentioned reducing and oxidizing agents are in separated
vessels but connected by leads and salt
bridge to allow electrical neutrality of the environment the redox
reaction can also occur. In this case the electron transfer from the reductant
to the oxidant runs through the lead and is named electric current This
experimental setup is called battery or galvanic cell. In suitable conditions
the electric current can feed many electric and electronic devices. If
an electric current is conveniently injected in opposition the battery
operation will be hindered or blocked or cause reverse operation if the
battery is rechargeable. While recharging proceeds, the synthesis of the
depleted chemical species on the former galvanic cell takes place on the
actual electrolytic cell.
The short lines above are a rough insight to the comprehension of redox reactions in living bodies. Nature in living bodies is characterized by high organization of consumption of chemical species, synthesis of chemical species in solution or in containers separated by selective membranes, electron receptors and donors, consumed and produced energies in good dosages in order to permit the physical manifestation of the living being. When material conditions are depleted due to external factors the defected organization decreases or breaks the physical manifestation of the living being. Energy supply and component substitution in good measure and just in time may restore the physical manifestation of the living being. On the other hand, when the living being destroys its own equilibrium and looses control and disorganizes the conditions to keep going then the physical manifestation of the living being stops. The living being will turn to exhibit physical manifestation when able to recognize and able to manage another organized and functional system if available.
Examples of oxidation and reduction reactions
a) Metallic zinc reduces copper ion to metallic copper.
![]()
b) Dichromate ion oxidizes an alcohol to a ketone.
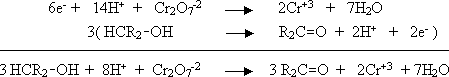
c) Nicotinamide adenine dinucleotide (NAD+ ) metabolizes ethanol to acetic aldehyde. Structural formulas of NAD+ and NADH can be selected by a click on the corresponding button on the upper right side of the page.
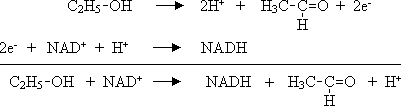
References
Any good book of general chemistry, organic chemistry and biochemistry.
Theme table
| Presentation |